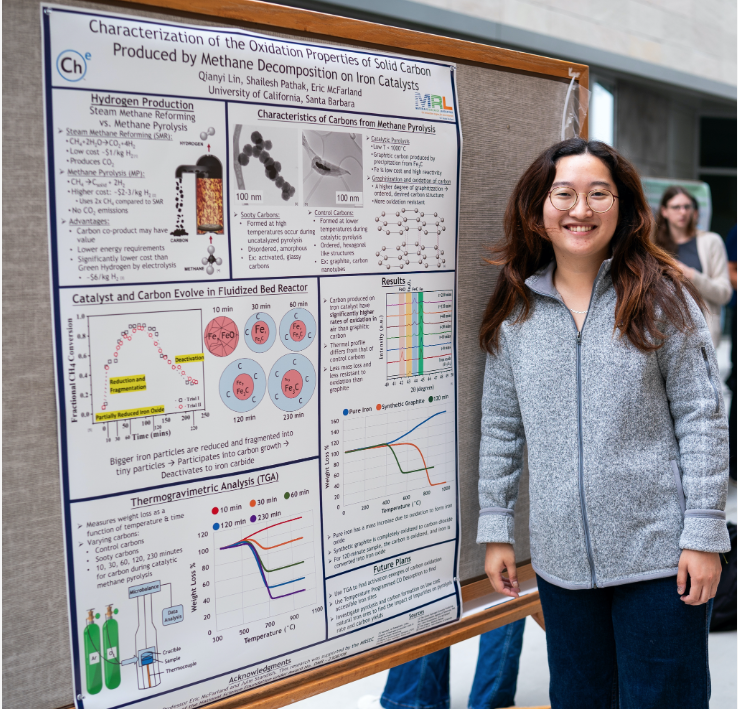
Hydrogen as a fuel will become important if it can be produced at a low cost and without carbon dioxide emissions. One method for producing hydrogen is methane pyrolysis which produces solid carbon and hydrogen without generating CO2. This process offers the potential for CO2-free hydrogen generation, making it an environmentally promising route for clean energy production. The produced carbons are of varying stability and characteristics depending on the type of carbon and degree of graphitization. For instance, the incomplete decomposition of methane could result in the synthesis of disordered carbon. In contrast, nickel-based catalysts at a lower temperature (~750°C) promote the synthesis of highly stable and ordered carbon nanotubes (CNTs). The knowledge of the thermal behavior oxidation properties of solid carbon is crucial as it affects the overall efficiency of the methane pyrolysis process. By understanding the properties, the process conditions can be tuned to produce more valuable carbon. When iron catalysts are used in a fluidized bed reactor carbon, it is deposited on the iron surface and can diffuse into the solid which stabilizes an iron carbide. The properties of the carbon deposited on the iron-based catalyst and the carbon-surface interface are unknown. In this work, insights into the temperature-dependent oxidation of the carbon can help better understand the carbon and the carbon-catalyst interactions. Using Thermogravimetric Analysis (TGA) over the temperature range 400°C to 1000°C, the oxidation properties of the carbon produced in the fluidized bed reactor have been characterized and compared to control carbons and the sooty carbons produced in the absence of a catalyst. The TGA spectra of the carbon produced on the iron catalyst are observed to exhibit significantly higher rates of oxidation in air than graphitic carbon and its thermal profile differs significantly from that of control carbons. Tuning the process conditions of methane pyrolysis can ensure a reliable method of creating valuable carbon and hydrogen which are useful for electricity production.